KOREAN
KOREAN

YUHANPROMOTES
BETTER HEALTH AND LIFE
Yuhan Chemical is doing its best to supply excellent APIs for the healthy lives of people around the world.


Our quality control department, which complies with cGMP regulation, meets the customer demands
and global regulatory requirements based on its extensive experience and state-of-the-art analytical instruments.
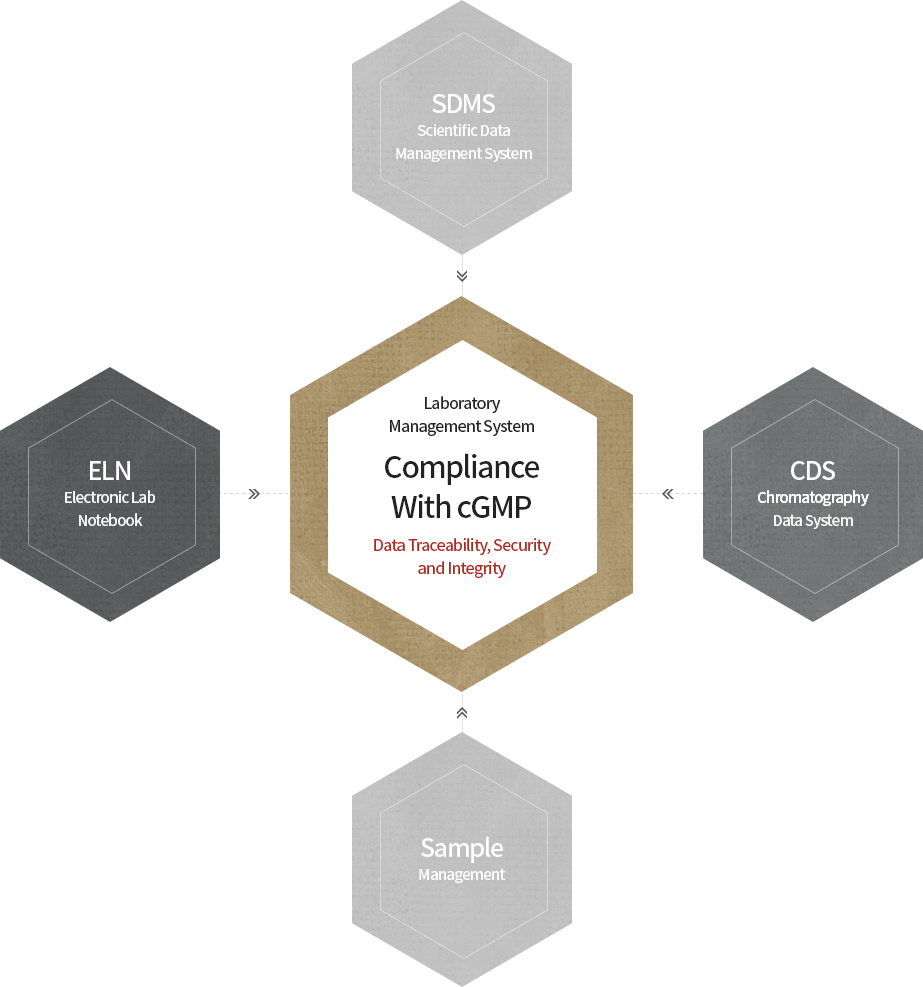
The analytical data generated by our QC laboratory is handled and stored through the networked CDS (Chromatography Data System) and
LMS (Laboratory Management System) to comply with global regulatory requirements on data integrity such as FDA 21 CFR Part 11.
NuGenesis as an LMS composed of ELN (Electronic Lab Notebook), SDMS (Scientific Data Management System) and Sample Management is used to automatically manage all the analytical data and information including issuing Certificates of Analysis (CoA).
![]() Yuhan Chemical Laboratory
Yuhan Chemical Laboratory
![]() Analytical Capabilities
Analytical Capabilities
- cGMP Compliant Quality Control Testing
- Stability Testing in compliance with ICH Q7 Guidelines
- Establishment of the Specifications and Test Methods for Raw materials, Intermediates and APIs
- Analytical Method Development and Validation
- Reference Standard Characterization
- Elemental Analysis and Trace Metals Testing
![]() Laboratory Instrumentation
Laboratory Instrumentation
- HPLC (UV, PDA, RI, Fluorescence, CAD)
- UPLC (UV, PDA Detectors)
- Ion Chromatograph
- LC/MS, LC/MSMS
- GC & Headspace (FID, TCD, ECD, NPD)
- GC/MS
- Prep-LC
- NMR Spectrometer
- X-ray Diffraction (XRD) System
- ICP/OES & ICP/MS
- Particle Size Analyzer
- DSC (Differential Scanning Calorimeter)
- Karl Fischer Titrator (Volumetric & Coulometric)
- UV Spectrophotometer
- FT/IR Spectrophotometer
- Raman Spectrophotometer
- Polarimeter
- TOC (Total Organic Carbon) Analyzer
- Refractometer
- Conductivity Meter
- Autotitrator